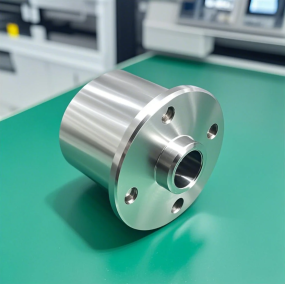
As we all know, medical device products are different from other automation or industry equipment, and the country has strict standards for control. Shenzhen EMAR Precision Technology, which is committed to precision machining of medical parts, shares with you the manufacturing standards for medical device products. Let's take a look together.
1、 Approval authority: Enterprises engaged in the operation of Class II and III medical devices shall report to the Provincial Drug Administration for review and approval, and be issued a "Medical Device Operation Enterprise License".
1. Provincial owned enterprises (registered with the Provincial Administration for Industry and Commerce) are directly accepted by the Provincial Drug Administration; 2. Other enterprises and units shall be accepted and preliminarily inspected by the drug regulatory departments of each city. If they pass the inspection, a written acceptance opinion shall be written and the preliminary inspection opinion shall be signed in the review form before being submitted to the provincial quality supervision bureau for approval.
2、 Declaration materials:
1. One application report;
2. Fill out three copies of the application form for the Medical Device Business License (photocopies are invalid);
3. One copy of the company's articles of association and the latest capital verification report (for certificate replacement, provide the company's balance sheet and income statement);
4. One copy of the enterprise self inspection summary (in accordance with the requirements of the "Implementation Rules for Qualification Recognition of Medical Device Operating Enterprises in Guangdong Province" and the "Guangdong Province Nuclear, Exchange, and Acceptance Standards");
5. A list of technical and maintenance personnel, along with one copy of their graduation certificate and one copy of their professional title certificate (stamped with the official seal of the unit);
6. One copy of property ownership certificate or lease agreement and floor plan for the operation and storage premises;
7. One detailed written initial review and acceptance report from the Municipal Drug Administration (excluding provincial enterprises);
8. One copy of the business name pre registration certificate or the copy of the "Corporate Business License";
9. Various management rules and regulations. a. Quality responsibility and veto power system; b. Warehouse acceptance, storage, and outbound review system; c. Quality analysis and feedback system; d. Validity management system; e. Sales quality management system for retail stores; f. Management system for special and imported medical devices; g. After sales service (installation, technical training, maintenance, repair, etc.) system; h. Quality tracking and adverse reaction reporting system for implanted and special medical devices; i. Return, non conformance, expiration date or elimination of medical devices processing report system; j. Health management system;
10. A self assurance statement regarding the authenticity of the information provided.
3、 Approval process
1. The provincial drug regulatory bureau can only accept applications if the application materials are complete and meet the requirements of the approval process. The handler shall organize relevant personnel or entrust the municipal drug regulatory department to conduct on-site acceptance within 15 working days from the date of acceptance, in accordance with the Implementation Rules for Qualification Recognition of Medical Device Operating Enterprises in Guangdong Province. The acceptance contents include: office, business, warehousing, and maintenance sites, testing and maintenance equipment and installation and maintenance records, quality system implementation, technical personnel on duty situation, regulations and rules on the types of medical devices operated and the collection and preservation of medical devices, quality standards for the types of medical devices operated, medical device product registration certificates and other relevant materials.
2. Those who pass the on-site acceptance shall fill out the medical device approval form within 10 working days, provide initial review opinions, and report to the department and bureau leaders for approval according to the procedures.
4、 Requirements for Declaration Materials
1. The application report should include: the economic nature of the enterprise, introduction of the main person in charge, department setting, branch setting, main business areas and main sales targets, main business varieties, specifications, storage facilities and surrounding environment.
2. List of technical and maintenance personnel: it refers to technical personnel in science and engineering, medicine, pharmacy and engineering. It is required to list the name, gender, age, final school of graduation, education background, major, technical title, company position and ID card number.
3. Plan of operating and storage facilities: The operating and storage facilities should indicate their length, width (meters), layout, shelf placement, location of fire equipment, and five prevention facilities.
4. All application materials must be printed on A4 paper and stamped with the official seal of the enterprise, unit, or superior supervisory unit. The enterprise name must be provided with a copy of the business registration certificate or the legal person's business license, as well as copies of the technical and maintenance personnel's graduation certificate, professional title certificate, etc. The applying unit should indicate on the copies that:; The copy matches the original; The words shall be stamped with the official seal and bound into a book in order.
5、 Other matters
Enterprises and units should follow the above approval procedures and requirements for declaration. It is strictly prohibited to give gifts of property to the handling personnel. If anyone requests or accepts property, please report to the Supervision Office of the Provincial Drug Administration.


 Spanish
Spanish Arabic
Arabic French
French Portuguese
Portuguese Belarusian
Belarusian Japanese
Japanese Russian
Russian Malay
Malay Icelandic
Icelandic Bulgarian
Bulgarian Azerbaijani
Azerbaijani Estonian
Estonian Irish
Irish Polish
Polish Persian
Persian Boolean
Boolean Danish
Danish German
German Filipino
Filipino Finnish
Finnish Korean
Korean Dutch
Dutch Galician
Galician Catalan
Catalan Czech
Czech Croatian
Croatian Latin
Latin Latvian
Latvian Romanian
Romanian Maltese
Maltese Macedonian
Macedonian Norwegian
Norwegian Swedish
Swedish Serbian
Serbian Slovak
Slovak Slovenian
Slovenian Swahili
Swahili Thai
Thai Turkish
Turkish Welsh
Welsh Urdu
Urdu Ukrainian
Ukrainian Greek
Greek Hungarian
Hungarian Italian
Italian Yiddish
Yiddish Indonesian
Indonesian Vietnamese
Vietnamese Haitian Creole
Haitian Creole Spanish Basque
Spanish Basque